Career opportunities
2ND YEAR OF THE MASTER'S DEGREE IN CLINICAL EVALUATION
Many career opportunities are available to students having successfully completed this academic year, particularly in the following fields:
- Pharmaceutical industries.
- Contracting companies (e.g. CROs).
- Academic groups in clinical research (e.g. Center for Clinical Investigation, CIC).
- Healthcare regulatory agencies (e.g. French National Agency for Medicines and Health Products, ANSM).
- Hospitals.
Examples of job titles:
- Clinical Project manager
- Clinical Development Manager
- Pharmacovigilance Manager
- Regulatory Affairs Specialist
- Medical writer
- Hospital Practitioner
- Study Coordinator
- Clinical Research Associate
- Medical Affairs Specialist
SURVEY ON OUR STUDENTS' CAREERS
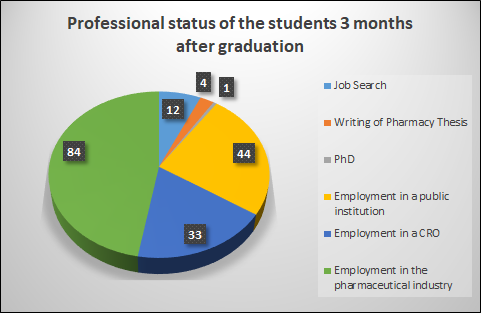
We have been able to establish the professional status of the 161 students graduated between 2011 and 2022, either using a follow-up survey, or by consulting their LinkedIn account.
Most of the students find a job less than three months after being graduated, mainly in the pharmaceutical industry, followed by the public sector and then the contract research organisations (CROs). A new phenomenon has been observed during the past years, that is to say students do not systematically search for a job after graduation: some take more time to write their pharmacy thesis, others travel during several months.
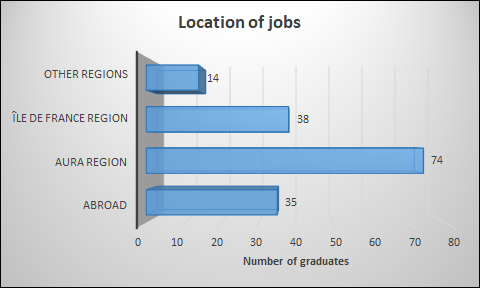
Most of the students find a job in the Auvergne-Rhône-Alpes (AURA) region and more particularly in Lyon and its suburbs. The region of Paris is a very attractive region too. Almost 22% of the graduates work abroad.